Understanding Nutrigenomics: How Genetic Variations Influence Nutrient Responses” is a fascinating article that delves into the field of nutrigenomics. It defines nutrigenomics as the study of how genetic variations influence responses to nutrients, highlighting how this knowledge can tailor dietary recommendations based on individual genetic profiles. The article also explores real-life examples of how nutrigenomic insights can inform dietary choices, emphasizing the role of personalized nutrition in achieving optimal health outcomes. Additionally, it provides practical tips for integrating nutrigenomic principles into daily dietary habits and discusses emerging trends in nutrigenomics research and technology. With its friendly tone and captivating introduction, this article is sure to engage readers and provide valuable insights into the exciting world of nutrigenomics.
This image is property of www.genosalut.com.
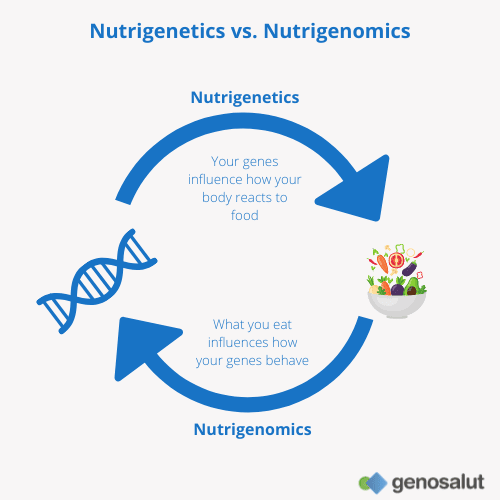
Understanding Nutrigenomics
Definition and explanation of nutrigenomics
Nutrigenomics, also known as nutritional genomics, is a field of study that examines how genetic variations influence an individual’s responses to nutrients. It explores how specific genes interact with nutrients and how these interactions impact various aspects of human health, including metabolism, disease risk, and overall well-being.
By understanding the relationship between genetics and nutrition, researchers and healthcare professionals can develop personalized dietary recommendations tailored to an individual’s genetic profile. This personalized approach takes into account an individual’s unique genetic makeup, allowing for more precise and effective nutrition interventions.
The role of nutrigenomics in dietary recommendation
Nutrigenomics plays a crucial role in shaping dietary recommendations by considering an individual’s genetic variations. Traditionally, dietary recommendations have been based on population-wide guidelines that may not take into account the specific genetic factors that influence nutrient metabolism and utilization in individuals.
With the advancement of nutrigenomics, it is now possible to analyze an individual’s genes and identify specific genetic variations that may affect nutrient requirements, absorption, and metabolism. This personalized approach allows for more accurate recommendations that take into consideration the individual’s unique genetic characteristics and dietary needs.
The importance of personalized nutrition
Personalized nutrition, which is informed by nutrigenomics, is vital for achieving optimal health outcomes. Each person’s genetic makeup is unique, and genetic variations can significantly impact how the body processes and utilizes nutrients. By tailoring dietary recommendations to an individual’s specific genetic profile, personalized nutrition can maximize the benefits of nutrients while minimizing any potential adverse effects.
Furthermore, personalized nutrition can be particularly beneficial in managing and preventing chronic diseases, such as cardiovascular diseases, diabetes, and certain types of cancer. By identifying genetic variations associated with increased disease risks or altered nutrient metabolism, personalized nutrition plans can be designed to mitigate these risks and optimize health outcomes.
Nutrigenomics in Action
Examples of nutrigenomic insights in dietary choices
Nutrigenomic insights can provide valuable information that guides individuals in making informed dietary choices. For example, genetic variations can influence how efficiently the body processes and utilizes certain nutrients, such as carbohydrates and fats. Individuals with specific genetic variations may have a higher risk of developing conditions like obesity or insulin resistance when consuming a high-carbohydrate or high-fat diet.
By understanding these genetic variations, individuals can make dietary choices that align with their genetic predispositions. For instance, individuals with a genetic predisposition to insulin resistance may benefit from reducing their carbohydrate intake and focusing on a diet that emphasizes healthier fats and proteins.
The impact of genetic variations on nutrient metabolism and dietary preferences
Genetic variations can significantly impact how the body metabolizes and utilizes nutrients. For example, some individuals may have genetic variations that affect the production of enzymes responsible for breaking down certain nutrients. This can result in differences in how efficiently the body processes and absorbs these nutrients, ultimately affecting overall health.
In addition to nutrient metabolism, genetic variations can also influence dietary preferences. Certain genes are associated with taste and food preferences. For example, variations in the TAS2R38 gene can impact an individual’s perception of bitter taste, leading to differences in food preferences and choices. Understanding these genetic variations can help individuals make dietary choices that align with their personal preferences and genetic predispositions.
The role of nutrigenomics in preventing and managing chronic diseases
Nutrigenomics has the potential to play a vital role in preventing and managing chronic diseases. By analyzing an individual’s genetic profile, healthcare professionals can identify genetic variations that increase the risk of developing certain diseases. These variations may affect nutrient metabolism, inflammation pathways, or antioxidant mechanisms, among other factors.
With this information, personalized nutrition plans can be developed to mitigate the effects of these genetic variants and reduce the risk of disease development or progression. For example, individuals with genetic variations associated with increased cardiovascular disease risk may benefit from dietary interventions that emphasize heart-healthy nutrients, such as omega-3 fatty acids or antioxidants.
Personalized Nutrition Strategies
Tips for integrating nutrigenomic principles into dietary habits
Integrating nutrigenomic principles into daily dietary habits can be a straightforward process with the right approach. Here are some tips to help individuals incorporate personalized nutrition strategies into their lifestyle:
Genetic testing: Consider undergoing genetic testing to gain insights into your genetic variations and understand how they may impact nutrient metabolism and dietary needs. This information can serve as a valuable foundation for personalized nutrition planning.
Consult with a professional: Seek guidance from a registered dietitian or healthcare professional experienced in nutrigenomics. They can help interpret your genetic results and translate them into practical dietary recommendations.
Tailor macronutrient ratios: Based on your genetic profile, customize the ratio of macronutrients (carbohydrates, proteins, and fats) in your diet. For example, individuals with genetic variations that affect carbohydrate metabolism may benefit from a lower carbohydrate intake and a higher emphasis on healthy fats and proteins.
Optimize nutrient absorption: Genetic variations can affect the body’s ability to absorb and utilize certain nutrients. Focus on food sources that maximize nutrient absorption, such as pairing vitamin C-rich foods with iron sources to enhance iron absorption.
Consider dietary supplements: Genetic variations can also impact an individual’s nutrient requirements. Discuss with a healthcare professional whether targeted supplementation may be necessary to address specific genetic variations associated with nutrient deficiencies.
Benefits of nutrition plans tailored to genetic profiles
Tailoring nutrition plans to the individual’s genetic profile offers several benefits:
Precision: Personalized nutrition accounts for the unique genetic variations of an individual, providing precise dietary recommendations based on their specific needs. This precision maximizes the potential health benefits and minimizes any potential adverse effects.
Improved compliance: When individuals understand the genetic influence on their nutrient responses, they are more likely to adhere to personalized nutrition plans. This improved compliance can lead to better health outcomes and long-term sustainable dietary habits.
Disease prevention and management: Personalized nutrition plans can be designed to target specific genetic variations associated with increased disease risks. By modifying dietary patterns and nutrient intake according to these genetic profiles, individuals can reduce the likelihood of developing chronic diseases or manage their existing conditions more effectively.
Optimization of nutrient utilization: By understanding an individual’s genetic variations, personalized nutrition plans can optimize nutrient utilization. This ensures that the body receives the necessary nutrients in the most efficient and effective way, enhancing overall health and well-being.
Addressing misconceptions and concerns about nutrigenomics
As nutrigenomics is a relatively new field, there can be misconceptions and concerns surrounding its implementation. It is essential to address these issues to provide a clear understanding of nutrigenomics and its potential benefits:
Limited genetic influence: Some individuals may believe that genetics play a minimal role in nutrition and that lifestyle factors alone determine health outcomes. However, while lifestyle factors are undoubtedly important, genetic variations can significantly impact nutrient responses and an individual’s dietary needs.
Genetic determinism: Another misconception is that genetic variations determine an individual’s health outcomes entirely. Nutrigenomics aims to provide insight into genetic predispositions, but it does not negate the influence of lifestyle choices. Personalized nutrition plans based on genetic profiles work in conjunction with lifestyle modifications to optimize health outcomes.
Accessibility and affordability: Concerns about the availability and affordability of nutrigenomic testing may arise. While the field is rapidly advancing, it is crucial to ensure equitable access to nutrigenomics resources to avoid exacerbating health disparities. Continued research and development are needed to make these resources more accessible to diverse populations.
By addressing these misconceptions and concerns, individuals can make informed decisions about nutrigenomics and recognize its potential to optimize their health and well-being.
Genes and Macronutrients
How genes affect absorption and utilization of macronutrients
Genes play a significant role in the absorption and utilization of macronutrients. Genetic variations can impact the production of enzymes and other proteins responsible for breaking down these macronutrients, affecting their metabolism and utilization in the body.
For example, genetic variations in the lactase gene influence lactase production, the enzyme responsible for digesting lactose, the sugar found in milk and dairy products. Individuals with variations that reduce lactase activity may experience symptoms of lactose intolerance, as they are unable to efficiently break down lactose.
Similarly, genetic variations can influence the production of enzymes involved in carbohydrate and fat metabolism. Some individuals may have genetic variants that affect insulin production or insulin sensitivity, leading to altered carbohydrate metabolism and an increased risk of conditions like diabetes or obesity.
Role of genetic variation in macronutrient preferences
Genetic variation also plays a role in macronutrient preferences, influencing an individual’s taste and food choices. Specific genes are involved in taste perception, such as bitter taste receptors and sweet taste receptors. Variations in these genes can impact an individual’s preference for certain macronutrients.
For example, the TAS1R2 and TAS1R3 genes are responsible for coding taste receptors that detect sweet flavors. Variations in these genes can influence an individual’s perception of sweetness, potentially impacting their preference for sweet-tasting foods. Similarly, genes involved in fat metabolism, such as the FFAR2 gene, can influence an individual’s preference for high-fat foods.
Understanding these genetic variations can help individuals make dietary choices that align with their genetic predispositions, optimizing nutrient utilization and overall health outcomes.
Importance of understanding the gene-macronutrient interaction
Understanding the interaction between genes and macronutrients is crucial for developing personalized nutrition recommendations. Genetic variations can significantly impact how individuals respond to different macronutrients, influencing nutrient metabolism, energy balance, and overall health.
By considering an individual’s genetic variations, nutrition professionals can tailor macronutrient recommendations to optimize metabolic efficiency and health outcomes. For example, individuals with genetic variations associated with reduced carbohydrate metabolism may benefit from consuming a diet that includes a higher proportion of healthy fats and proteins while moderating carbohydrate intake.
Additionally, understanding the gene-macronutrient interaction can provide insights into the biological mechanisms underlying certain dietary patterns and their effects on health. This knowledge can contribute to the development of evidence-based dietary guidelines that consider an individual’s genetic variations, moving toward a more personalized and precise approach to nutrition.

This image is property of europepmc.org.
Nutrigenomic Testing
Understanding nutrigenomic testing
Nutrigenomic testing involves analyzing an individual’s genetic material, typically through a saliva or blood sample, to identify genetic variations related to nutrient metabolism and dietary responses. The genetic information obtained from this testing can provide insight into an individual’s unique genetic profile, allowing for a more personalized approach to nutrition.
Nutrigenomic testing usually targets specific genes or variations known to impact nutrient metabolism, absorption, and utilization. These variations are then interpreted in the context of current scientific knowledge to provide personalized nutrition recommendations tailored to an individual’s genetic profile.
The benefits and limitations of nutrigenomic testing
Nutrigenomic testing offers several benefits:
Personalized recommendations: By identifying an individual’s genetic variations, nutrigenomic testing provides personalized nutrition recommendations based on their unique genetic profile. This targeted approach can optimize nutrient utilization and overall health outcomes.
Precision and specificity: Nutrigenomic testing helps identify specific genetic variations associated with nutrient metabolism and dietary responses. This precision allows for more accurate dietary recommendations compared to population-wide guidelines.
Increased motivation and adherence: Nutrigenomic testing can enhance individuals’ motivation and adherence to personalized nutrition plans. Understanding the genetic influence on nutrient responses can empower individuals to make more informed dietary choices and maintain long-term lifestyle changes.
However, nutrigenomic testing also has limitations that need to be considered:
Complexity of gene-nutrient interactions: Nutrigenomic testing provides valuable insights into an individual’s genetic profile, but the relationship between genes and nutrition is complex. There are numerous genes involved in nutrient metabolism, and genetic variations can interact with environmental factors, making it challenging to predict precise dietary responses solely based on genetic information.
Interpretation challenges: Interpreting genetic data requires expertise and a deep understanding of nutrigenomics. Not all genetic variations have well-established associations with nutrient responses or health outcomes, which can make it challenging to provide accurate and actionable recommendations based solely on genetic information.
Limited evidence base: Nutrigenomics is a rapidly evolving field, and some scientific knowledge regarding gene-nutrient interactions is still emerging. The evidence base for certain genetic variations and their impact on nutrition is evolving, and additional research is needed to further validate and refine personalized nutrition recommendations.
Future innovations in nutrigenomic testing
The field of nutrigenomic testing is continuously evolving, and ongoing research and technological advancements hold promise for future innovations. Some potential areas of development include:
Increased genome coverage: As sequencing technologies continue to advance, it becomes more feasible to perform whole-genome sequencing, providing a comprehensive analysis of an individual’s genetic variations. This expanded genome coverage can offer a more detailed understanding of gene-nutrient interactions and enable greater personalization of nutrition recommendations.
Integration of other omics data: Nutrigenomic testing may integrate genomic data with other omics data, such as epigenomics, transcriptomics, and metabolomics. These additional layers of information can provide a more holistic understanding of how genetic variations influence nutrient responses and health outcomes.
Digital platforms and AI: The integration of digital platforms and artificial intelligence (AI) technologies may enhance nutrigenomic testing and its application in personalized nutrition recommendations. AI algorithms can analyze large datasets and identify patterns that may not be apparent to human researchers, allowing for more accurate interpretation of genetic data and personalized dietary guidance.
Longitudinal studies: Longitudinal studies that follow individuals over time can provide valuable insights into the long-term effects of gene-nutrient interactions and personalized nutrition interventions. These studies can help refine and validate the effectiveness of nutrigenomic testing and its impact on health outcomes.
By leveraging these future innovations, nutrigenomic testing can continue to evolve and improve, offering more precise, practical, and evidence-based personalized nutrition recommendations.
The Role of Micronutrients
Understanding the role of micronutrients in the body
Micronutrients, such as vitamins and minerals, play essential roles in maintaining optimal health and well-being. Each micronutrient has specific functions in the body, supporting various physiological processes and contributing to overall vitality.
For example, vitamin C is crucial for collagen synthesis and immune function, while iron is necessary for oxygen transport in the blood and energy production. Similarly, minerals like calcium are essential for bone health, while zinc is involved in numerous enzymatic reactions and immune system function.
Adequate intake of micronutrients is necessary to prevent deficiencies and optimize health. Genetic variations can influence the body’s requirements and utilization of these micronutrients, making personalized nutrition recommendations valuable for addressing individual needs.
How genetic variations affect micronutrient needs
Genetic variations can influence an individual’s micronutrient needs and utilization. Certain genetic variations can alter the absorption, metabolism, or utilization of specific micronutrients, potentially increasing the risk of deficiencies or affecting overall health outcomes.
For example, variations in the SLC23A1 gene can impact the body’s ability to absorb and utilize vitamin C efficiently. Individuals with these variations may require a higher intake of vitamin C to meet their body’s needs adequately.
Similarly, genetic variations in the HFE gene can affect iron metabolism and increase the risk of iron overload or iron deficiency. Understanding these genetic variations can help tailor micronutrient recommendations to address individual needs and optimize health outcomes.
Implications of micronutrient deficiencies
Micronutrient deficiencies can have significant health implications, leading to a range of symptoms and complications. For example:
Iron deficiency can result in anemia, fatigue, and impaired cognitive function.
Vitamin D deficiency is associated with weakened bones, an increased risk of fractures, and impaired immune function.
Zinc deficiency can impair wound healing, weaken the immune system, and lead to dermatological issues.
These are just a few examples, and various micronutrient deficiencies can manifest with different symptoms and health consequences. Genetic variations can contribute to an individual’s susceptibility to these deficiencies and influence their nutritional needs.
Understanding an individual’s genetic profile allows for personalized recommendations that address specific micronutrient requirements and mitigate the risk of deficiencies. By tailoring dietary strategies to genetic variations, personalized nutrition can optimize micronutrient intake and support overall health and well-being.

This image is property of d3i71xaburhd42.cloudfront.net.
The Influence of Nutrigenomics on Disease Risks
The relation between nutrient-gene interactions and disease risks
The interplay between nutrients and genes can significantly influence disease risks. Genetic variations can impact nutrient metabolism, absorption, utilization, and other biological processes, potentially altering disease susceptibility or progression.
For example, variations in the FTO gene have been associated with increased obesity risk. The FTO gene influences appetite regulation and energy expenditure, and specific variations can contribute to an increased preference for energy-dense foods and reduced feelings of satiety.
Similarly, genetic variations in the APOE gene are associated with different risks for developing cardiovascular disease and Alzheimer’s disease. Certain variations in this gene can influence lipid metabolism and cholesterol levels, affecting cardiovascular health.
By understanding these nutrient-gene interactions, personalized nutrition interventions can be designed to mitigate these genetic risks and decrease disease susceptibility.
Examples of specific diseases influenced by nutrient-gene interactions
Numerous diseases can be influenced by nutrient-gene interactions. Here are a few examples:
Cardiovascular Disease: Genetic variations can impact lipid metabolism, blood pressure regulation, inflammation pathways, and other cardiovascular risk factors. Nutrigenomic approaches can help identify individuals with increased cardiovascular disease risk based on their genetic profile, allowing for tailored dietary interventions to mitigate these risks.
Diabetes: Genetic variations can influence insulin production, insulin sensitivity, and glucose metabolism. Understanding an individual’s genetic predispositions can guide personalized nutrition strategies that optimize blood sugar control and reduce the risk of developing diabetes.
Cancer: Certain genetic variations can impact an individual’s susceptibility to specific types of cancer. Nutrigenomic approaches can identify genetic variants associated with increased cancer risks and guide dietary recommendations to lower those risks.
These examples illustrate how nutrigenomics can enable targeted interventions that address genetic predispositions, helping individuals reduce their risk of developing these diseases and potentially manage existing conditions more effectively.
The role of personalized nutrition in disease prevention
Personalized nutrition, informed by nutrigenomics, plays a vital role in disease prevention. By identifying an individual’s genetic variations, personalized nutrition interventions can be designed to mitigate the effects of these genetic risks and optimize health outcomes.
Tailored dietary recommendations can address genetic factors associated with increased disease risks, such as altered nutrient metabolism, inflammation pathways, or antioxidant mechanisms. Nutrigenomic approaches allow for precise interventions that target the underlying genetic predispositions, potentially reducing the likelihood of developing chronic diseases and improving overall health.
It is important to note that personalized nutrition is not a standalone treatment or prevention method. Lifestyle factors, such as physical activity, stress management, and adequate sleep, also play critical roles in disease prevention and management. Personalized nutrition should always be integrated into a comprehensive approach to overall well-being.
Ethical Aspects of Nutrigenomics
The ethical considerations in nutrigenomics
As with any scientific field, nutrigenomics raises ethical considerations that need to be addressed. Some key ethical considerations include:
Informed consent: Nutrigenomic testing involves the collection and analysis of an individual’s genetic information. It is essential to ensure informed consent, clearly explaining the purpose, potential benefits, and limitations of testing, as well as the management of personal genetic data.
Privacy and data protection: Nutrigenomic testing generates sensitive genetic information. Strict privacy and data protection measures must be in place to protect individuals’ genetic data from unauthorized access, misuse, or discrimination.
Genetic counseling: Genetic counseling is an important ethical consideration, particularly when nutrigenomic testing identifies genetic variations associated with increased disease risks. Genetic counselors can help individuals understand the implications of their genetic information, interpret the results, and make informed decisions about their health.
Equity and access: Ensuring equitable access to nutrigenomics resources is crucial to prevent exacerbating health disparities. Efforts should be made to make nutrigenomic testing and personalized nutrition recommendations accessible and affordable to diverse populations to avoid further perpetuating healthcare inequalities.
These ethical considerations guide responsible and equitable practices in nutrigenomics research and clinical implementation, promoting transparency, privacy, and individuals’ rights.
Privacy and data protection concerns
Privacy and data protection are paramount in nutrigenomics, given the sensitive nature of genetic information. Several privacy and data protection concerns need to be addressed to ensure individuals’ rights are respected:
Informed consent: Individuals undergoing nutrigenomic testing should provide informed consent, clearly understanding how their genetic data will be used, stored, and protected.
Anonymization and de-identification: Genetic data should be adequately anonymized and de-identified to protect individuals’ privacy. The data should not be linked directly to personal identifiers whenever possible.
Secure storage and transmission: Genetic data should be stored and transmitted using secure encryption methods and protected by robust firewalls to prevent unauthorized access.
Access restrictions: Only authorized personnel should have access to individuals’ genetic data, and appropriate measures should be in place to control access and minimize the risk of data breaches.
Non-discrimination: The use of genetic information for discriminatory purposes, such as employment or insurance decisions, should be strictly prohibited to protect individuals’ rights and prevent genetic discrimination.
It is essential for researchers, healthcare professionals, and policymakers to prioritize privacy and data protection in nutrigenomics, fostering trust and ensuring individuals’ rights are upheld.
Equitable access to nutrigenomics resources
Equitable access to nutrigenomics resources is vital to avoid exacerbating health disparities. Nutrigenomics should be accessible to diverse populations, regardless of socioeconomic status, race, or geographic location.
Efforts should be made to:
Promote diversity in research: Include diverse populations in nutrigenomics research to ensure that personalized nutrition recommendations are applicable and effective for individuals from various ethnic and cultural backgrounds.
Reduce cost barriers: Nutrigenomic testing and associated services should be made affordable and accessible to individuals of all socioeconomic backgrounds. Public health initiatives and insurance coverage can play a role in ensuring these resources are within reach for all.
Health education and literacy: Promote health education and literacy programs that provide clear information about the benefits and limitations of nutrigenomics. This empowers individuals to make informed decisions about their health and access relevant resources.
Collaboration and partnerships: Establish collaborations between researchers, healthcare professionals, policymakers, and community leaders to develop initiatives that address health disparities and provide equitable access to nutrigenomics resources.
By prioritizing equitable access and addressing health disparities, nutrigenomics can contribute to improved health outcomes for all individuals, promoting inclusivity and fairness.

This image is property of www.frontiersin.org.
The Future of Nutrigenomics
Emerging trends in nutrigenomics research
Nutrigenomics research is advancing rapidly, and several emerging trends are shaping the future of the field:
Multi-omics integration: Integrating genetic data with other omics data, such as epigenomics, transcriptomics, and metabolomics, can provide a more comprehensive understanding of gene-nutrient interactions. These multi-omics approaches offer deeper insights into the mechanisms underlying nutrient responses and health outcomes.
Microbiome-nutrigenomics interactions: The gut microbiome plays a critical role in nutrient metabolism, absorption, and overall health. Research exploring the interplay between the gut microbiome and nutrigenomics is expanding, uncovering potential connections between microbial diversity, genetic variations, and dietary responses.
Gene editing and precision interventions: Advances in gene editing technologies, such as CRISPR-Cas9, hold promise for precision interventions in nutrigenomics. These technologies allow for targeted modifications of specific genetic variations, potentially correcting or mitigating the effects of genetic risks associated with nutrient metabolism.
Digital platforms and mobile apps: Digital platforms, mobile apps, and wearable devices have the potential to enhance the delivery of personalized nutrition recommendations. These technologies can integrate genetic data, health information, and lifestyle tracking, providing real-time feedback and guidance for individuals to optimize their dietary habits.
Potential of nutrigenomic testing in personalized nutrition recommendations
Nutrigenomic testing has the potential to play a prominent role in personalized nutrition recommendations. As genetic testing becomes more accessible and affordable, incorporating an individual’s genetic profile into nutrition assessments can provide valuable insights for tailored dietary recommendations.
By analyzing an individual’s genetic variations, healthcare professionals can identify specific genetic risks or nutrient-gene interactions that may influence nutrient metabolism or disease susceptibility. This information can guide the creation of personalized nutrition plans, optimizing nutrient intake and addressing individual nutrient requirements.
Moreover, nutrigenomic testing can help identify individuals who may benefit from targeted dietary interventions, such as those with specific genetic variations associated with nutrient deficiencies or disease risks. This personalized approach can enhance the precision and effectiveness of nutrition interventions, promoting better health outcomes.
Challenges of widespread adoption of nutrigenomics
Despite the promising potential of nutrigenomics, several challenges need to be addressed for its widespread adoption:
Education and awareness: Nutrigenomics is a relatively new field, and there is a need to increase education and awareness among healthcare professionals, researchers, and the general public. Providing accurate and accessible information is crucial for promoting understanding and acceptance of nutrigenomics.
Standardization of protocols: Standardization of nutrigenomic testing protocols, data interpretation, and reporting guidelines is essential to ensure consistency and reliability across different laboratories and practitioners. Harmonizing practices can enhance the quality and reproducibility of nutrigenomics research and clinical applications.
Evidence-based guidelines: Developing evidence-based guidelines for the integration of nutrigenomics in clinical practice is crucial. Continued research and validation are necessary to establish reliable associations between genetic variations, nutrient responses, and health outcomes, providing a solid foundation for dietary recommendations.
Accessibility and affordability: Ensuring widespread access to nutrigenomic testing and personalized nutrition resources is essential to prevent further health disparities. Efforts should be made to improve affordability, expand insurance coverage, and increase availability in diverse healthcare settings.
By addressing these challenges, nutrigenomics can continue to progress and achieve widespread adoption, empowering individuals to optimize their health through personalized nutrition strategies.
Conclusion
Understanding nutrigenomics and its implications is crucial for achieving optimal health outcomes. Nutrigenomics explores the relationship between genetic variations, nutrient responses, and disease risks, providing insight into how personalized nutrition can be tailored to an individual’s genetic profile.
By integrating nutrigenomic principles into dietary habits, individuals can optimize nutrient utilization, support overall well-being, and reduce the risk of developing chronic diseases. Personalized nutrition strategies based on nutrigenomics offer precise, evidence-based recommendations that account for an individual’s unique genetic variations and dietary needs.
While nutrigenomics is evolving rapidly, challenges such as ethical considerations, privacy protection, equitable access, and the need for further research and standardization must be addressed. By overcoming these challenges, nutrigenomics can continue to advance, offering improved personalized nutrition recommendations, revolutionizing healthcare, and supporting individuals in achieving their health and wellness goals.
In conclusion, nutrigenomics has the potential to revolutionize nutrition science and transform individuals’ health outcomes by harnessing the power of their genetic information. By embracing personalized nutrition strategies and integrating insights from nutrigenomics, individuals can optimize their dietary habits, prevent and manage chronic diseases, and work towards achieving their full health potential.



