In the article “The Impact of Malnutrition on Epigenetics: Exploring the Relationship between Genetics and Nutrition”, we delve into the intricate relationship between genetics and nutrition, with a specific focus on how malnutrition affects epigenetics. Nutrigenomics, a field that explores the influence of genetics on individual responses to diet, highlights the significance of personalized nutrition in optimizing health outcomes. By understanding how genetic variations impact dietary responses, we can uncover the role of gene expression and epigenetics in shaping personalized nutrition strategies. From health implications to disease prevention and management, and even performance enhancement, nutrigenomics opens up new possibilities for tailoring dietary interventions based on individual genetic profiles. However, challenges and limitations such as ethical considerations, access and affordability, and the complexity of gene-diet interactions must also be addressed. Looking ahead, advancements in technology, integration with healthcare, and public education and awareness offer promising opportunities for the future of nutrigenomics. Understanding the impact of malnutrition on epigenetics is crucial in optimizing individual health outcomes and revolutionizing our approach to personalized health and nutrition.
This image is property of www.mdpi.com.
Understanding Epigenetics
Defining Epigenetics
Epigenetics refers to the study of changes in gene expression or activity that do not involve alterations to the underlying DNA sequence. These changes can be influenced by various factors, including environmental factors, lifestyle choices, and nutrition. Epigenetic modifications, such as DNA methylation and histone modifications, can affect how genes are turned on or off, leading to differences in gene expression and potentially impacting health and disease outcomes.
Importance of Epigenetics in Health and Disease
Epigenetics plays a crucial role in regulating gene expression, which in turn affects various biological processes in the body. By modulating the activity of certain genes, epigenetic modifications can influence the development and progression of diseases, including cancer, cardiovascular diseases, and neurological disorders. Understanding epigenetics is essential for unraveling the complexity of gene regulation and identifying potential therapeutic targets for disease prevention and treatment.
Key Concepts: Gene Expression and Epigenetic Modifications
Gene expression refers to the process by which genetic information encoded in DNA is used to produce functional molecules, such as proteins. Epigenetic modifications, on the other hand, are chemical changes to the DNA or histone proteins that can influence gene expression. DNA methylation, for example, involves the addition of a methyl group to a DNA molecule, which can reduce gene expression. Histone modifications, including acetylation and methylation, can also impact gene expression by altering the structure of chromatin and making genes more or less accessible to the transcriptional machinery. These changes in gene expression are crucial for normal development, as well as for maintaining the proper functioning of various tissues and organs in the body.
The Role of Nutrition in Epigenetics
Effect of Nutrients on Gene Expression
Nutrients obtained from the diet play a vital role in epigenetic regulation by providing the necessary building blocks for DNA methylation and histone modifications. For example, folate, vitamin B12, and other methyl-donor nutrients are essential for DNA methylation reactions. Insufficient intake of these nutrients can result in altered DNA methylation patterns and gene expression profiles. Similarly, certain dietary components, such as polyphenols found in fruits and vegetables, can modulate histone modifications and influence gene expression. The effect of nutrients on gene expression highlights the importance of a balanced and nutrient-rich diet for maintaining optimal epigenetic regulation.
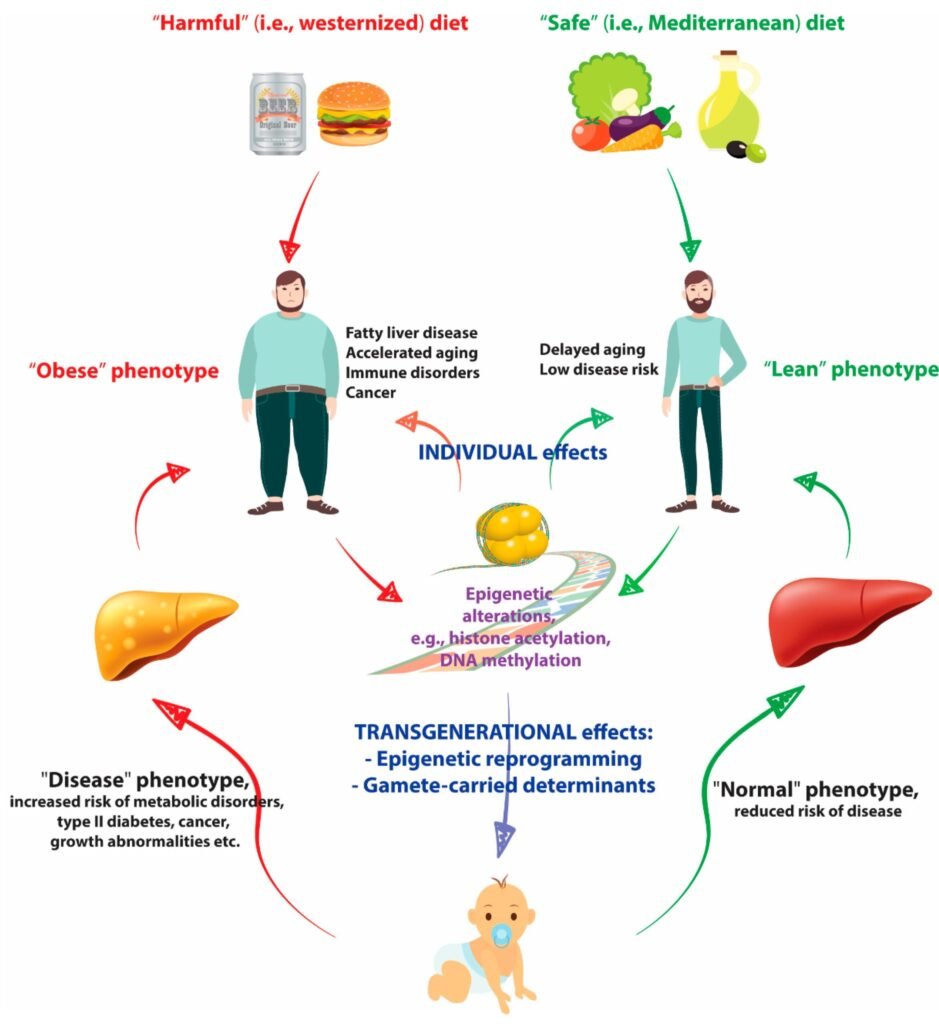
Impact of Malnutrition on Epigenetic Changes
Malnutrition, whether due to inadequate intake or poor absorption of nutrients, can have detrimental effects on epigenetic regulation. In cases of severe malnutrition, such as protein-energy malnutrition or micronutrient deficiencies, the body may prioritize essential functions, leading to alterations in DNA methylation and histone modifications. These epigenetic changes can have long-lasting effects on gene expression, potentially contributing to the development of various health conditions. It is crucial to address malnutrition and ensure adequate nutrient intake to support proper epigenetic regulation and overall health.
Microbial Influence on Epigenetics
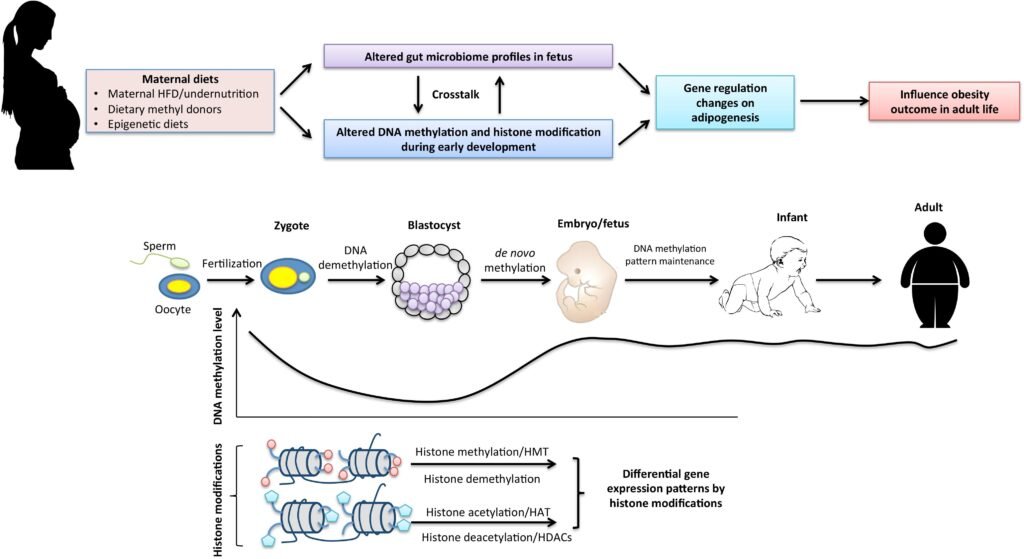
The human body harbors trillions of microbes, collectively known as the microbiota, which interact with the host and influence various physiological processes, including epigenetic regulation. The gut microbiota, in particular, plays a crucial role in nutrient metabolism and absorption, and can produce metabolites that influence epigenetic modifications. Short-chain fatty acids, for example, produced by certain gut bacteria, can act as histone deacetylase inhibitors, affecting histone modifications and gene expression. The interplay between the microbiota and host epigenetics highlights the complex relationship between nutrition, microbes, and host physiology.
Exploring Nutrigenomics
Defining Nutrigenomics
Nutrigenomics is the study of how genetic variations influence an individual’s response to diet and how dietary components can modulate gene expression and influence health outcomes. It aims to understand the interplay between diet, genes, and health by exploring the molecular mechanisms underlying gene-diet interactions. Nutrigenomics seeks to provide personalized nutrition recommendations based on an individual’s genetic profile to optimize health outcomes and prevent or manage diseases.
State of the Current Research in Nutrigenomics
The field of nutrigenomics is rapidly advancing, with a growing body of research exploring the relationship between genetics and nutrition. Current studies employ various techniques, including genome-wide association studies (GWAS), epigenome-wide association studies (EWAS), and transcriptome analyses to identify genetic variations associated with dietary responses and health outcomes. These studies have already uncovered genetic variants that influence nutrient metabolism, taste perception, and susceptibility to diet-related diseases. However, further research is needed to fully understand the complex gene-diet interactions and translate these findings into practical applications for personalized nutrition.
Interplay between Diet, Genes, and Health Outcomes
Nutrigenomics highlights the intricate interplay between diet, genetic variations, and health outcomes. Genetic variations can affect an individual’s ability to metabolize certain nutrients or respond to dietary interventions. For example, variations in genes involved in folate metabolism can impact an individual’s response to dietary folate intake and influence their risk for neural tube defects or cardiovascular diseases. Similarly, genetic variants in taste receptors can influence preferences for sweet or bitter foods, potentially impacting food choices and dietary patterns. Understanding these gene-diet interactions is essential for tailoring personalized dietary interventions to optimize health outcomes and prevent or manage diet-related diseases.
Implications of Malnutrition on Epigenetics
Short-term and Long-term Consequences
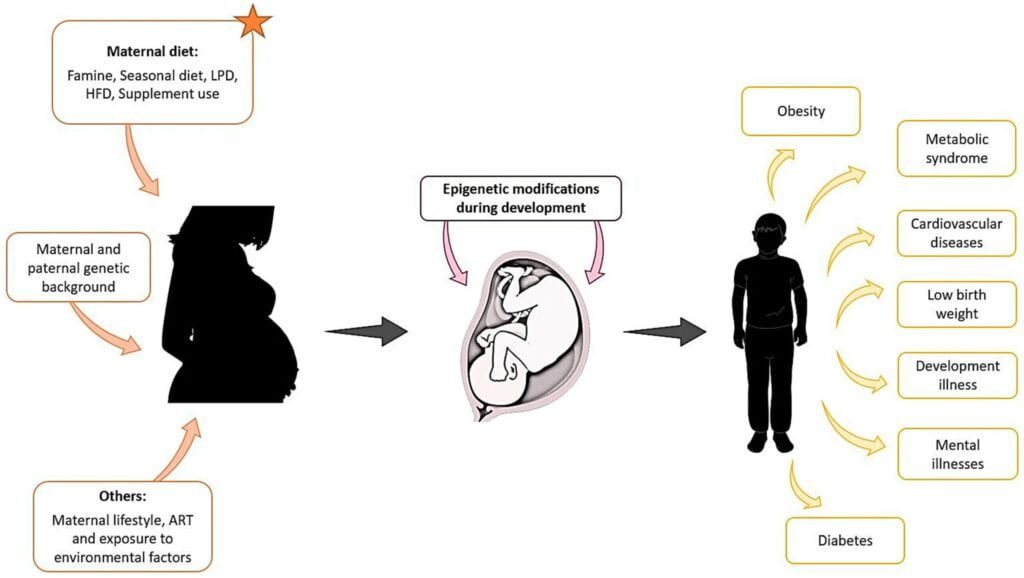
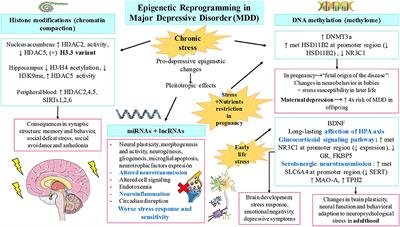
Malnutrition, particularly during critical periods of development, can have both short-term and long-term consequences on epigenetic regulation. Inadequate nutrient intake during pregnancy, infancy, or early childhood can lead to epigenetic changes that persist throughout life, affecting gene expression and increasing the risk of various diseases. These epigenetic modifications can impact not only the individual but also future generations, as some epigenetic marks can be inherited. It is crucial to address malnutrition early in life to minimize the long-term health implications and break the cycle of intergenerational malnutrition.
Specific Considerations for Early Life Nutrition
Nutrition during early life, including the prenatal and postnatal periods, has a profound influence on epigenetic regulation and long-term health outcomes. Adequate intake of essential nutrients during pregnancy is crucial for proper fetal development and establishing healthy epigenetic profiles. Breastfeeding, too, provides unique nutritional and immunological benefits, supporting optimal epigenetic regulation and development. Early-life nutrition can program gene expression patterns and metabolic pathways, impacting the risk of obesity, cardiovascular diseases, and other conditions later in life. Therefore, ensuring proper nutrition during these critical periods is vital for optimizing epigenetic regulation and minimizing the risk of chronic diseases.
Epigenetics and Obesity: Interplay Between Genetics and Malnutrition
Epigenetics plays a significant role in the development and progression of obesity, a complex condition influenced by both genetic and environmental factors. An individual’s genetic background can influence their susceptibility to weight gain and metabolic dysregulation in response to a high-calorie diet or sedentary lifestyle. At the same time, malnutrition, especially excessive calorie intake or a high-fat diet, can induce epigenetic changes that contribute to obesity. Increased DNA methylation and altered histone modifications can affect genes involved in energy metabolism, appetite regulation, and fat storage, leading to an imbalance in energy homeostasis and the development of obesity. Understanding the interplay between genetics, malnutrition, and epigenetics is critical for developing effective prevention and treatment strategies for obesity.
This image is property of www.mdpi.com.
Health Implications from Nutrigenomic Studies
Customization of Dietary Plans
Nutrigenomic studies enable the customization of dietary plans based on an individual’s unique genetic profile. By identifying genetic variations that influence nutrient metabolism, satiety, taste perception, or responses to dietary interventions, personalized nutrition recommendations can be developed to optimize health outcomes. For example, individuals with genetic variations associated with poor folate metabolism may benefit from higher folate intake or supplementation to support proper DNA methylation and reduce the risk of neural tube defects. Customizing dietary plans based on genetic information can enhance the effectiveness of nutrition interventions and improve health outcomes.
Targeted Dietary Interventions for Preventing Disease
Nutrigenomic research has the potential to inform targeted dietary interventions for preventing and managing various diseases. By identifying genetic variants associated with increased disease risk or altered responses to specific dietary components, personalized dietary recommendations can be developed to mitigate these risks. For instance, individuals carrying genetic variations associated with impaired lipid metabolism may benefit from a diet low in saturated fats and high in unsaturated fats to minimize the risk of cardiovascular diseases. Targeted dietary interventions based on an individual’s genetic background can help prevent disease development, optimize treatment outcomes, and improve overall health.
Performance Enhancement Through Nutrigenomics
Understanding the genetic variations that influence athletic performance and recovery can help athletes optimize their nutrition strategies for enhanced performance. Nutrigenomic studies have identified genetic variants impacting factors such as muscle function, nutrient utilization, and inflammation response, which can influence an athlete’s response to specific nutrients or dietary strategies. By tailoring their nutrition plans to their genetic predispositions, athletes can optimize energy metabolism, support muscle recovery, and enhance endurance. Nutrigenomics has the potential to revolutionize sports nutrition, enabling athletes to achieve their full potential and improve their competitive performance.
Challenges in the Field of Nutrigenomics
Ethical Issues Around Genetic Information Use
The use of genetic information in personalized nutrition raises various ethical concerns. Privacy and data protection are paramount, as genetic information is highly sensitive and has implications beyond nutrition. Safeguarding individuals’ genetic data, ensuring informed consent, and implementing strict data management protocols are essential to address these ethical concerns. Additionally, concerns about genetic determinism and stigmatization may arise if genetic information is misinterpreted or used inappropriately. Ethical guidelines and regulations should be in place to govern the use of genetic information in nutrigenomics and protect individuals’ rights and well-being.
Addressing Accessibility and Affordability
Making nutrigenomic testing and personalized nutrition services accessible to diverse populations is a significant challenge. Genetic testing and interpretation can be expensive, limiting access to those with financial means. Additionally, there is a lack of standardization and harmonization in nutrigenomic research, making it challenging to translate findings into practical applications. Efforts should be made to address these challenges by promoting collaboration and knowledge sharing, reducing costs, and developing guidelines for incorporating nutrigenomics into healthcare practice. Ensuring equitable access to personalized nutrition is crucial for maximizing its potential benefits.
Complexity of Gene-Diet Interactions
Deciphering the complex interplay between genes and dietary factors is a major challenge in nutrigenomic research. The relationship between specific genetic variations and dietary responses is multifaceted and influenced by various factors, including the overall diet, environmental influences, and gene-environment interactions. Additionally, the effects of specific dietary components or interventions can vary based on an individual’s genetic background and other factors. Understanding these interactions requires comprehensive and multidisciplinary research approaches, incorporating genetics, nutrition, bioinformatics, and other fields. Overcoming these complexities will improve the accuracy and reliability of personalized nutrition recommendations and advance the field of nutrigenomics.
This image is property of www.frontiersin.org.
Advancements in Nutrigenomic Research
New Technologies Revolutionizing Nutrigenomics
Advancements in technology have greatly facilitated nutrigenomic research. Next-generation sequencing techniques, such as whole-genome sequencing and targeted sequencing panels, enable the identification of genetic variations associated with dietary responses and health outcomes on a larger scale. These techniques provide a comprehensive view of an individual’s genetic profile and allow for the exploration of rare and common genetic variants. Additionally, advances in high-throughput omics technologies, including genomics, transcriptomics, and metabolomics, allow for the simultaneous analysis of multiple biomarkers and the assessment of their interactions. These technological advancements have the potential to uncover new genetic associations, enhance our understanding of gene-diet interactions, and improve personalized nutrition strategies.
Artificial Intelligence Potential in the Field
Artificial intelligence (AI) holds significant promise in nutrigenomics research and personalized nutrition. AI algorithms can analyze large-scale genomic and phenotypic data, identifying patterns and associations that may not be evident to human researchers. The integration of AI with nutrigenomics can enable the development of predictive models for individual dietary responses, helping to tailor nutrition recommendations based on genetic profiles more accurately. AI can also assist in data interpretation, predicting disease risk, and identifying optimal dietary interventions. However, ethical considerations, such as data privacy and transparency, must be carefully addressed when incorporating AI into nutrigenomics research and practice.
Innovations in Methodologies
In addition to technological advancements, innovations in research methodologies are shaping the field of nutrigenomics. Multi-omics approaches, combining genomics, transcriptomics, epigenomics, and other omics data, provide a more comprehensive understanding of the complex mechanisms underlying gene-diet interactions. Integration of multi-omics data with clinical and dietary data can enhance our ability to identify relevant genetic variants and their impact on health outcomes. Additionally, longitudinal studies and large-scale cohort studies can provide valuable insights into the long-term effects of gene-diet interactions on health and disease. These innovative methodologies contribute to building a more comprehensive and accurate understanding of nutrigenomics and its implications for personalized nutrition.
Nutrigenomics and Mainstream Healthcare
Integrating Nutrigenomics in Healthcare Practices
The integration of nutrigenomics into mainstream healthcare practices is an essential step in realizing its potential for personalized nutrition. This integration requires collaboration between geneticists, nutritionists, and healthcare providers to ensure the seamless integration of genetic information into clinical practice. Electronic health records and decision support systems can facilitate the incorporation of genetic data into patient care, enabling clinicians to provide tailored nutrition recommendations based on an individual’s genetic profile. Education and training for healthcare professionals are also crucial to ensure their competence in understanding and applying nutrigenomic principles.
Revolutionizing Preventive Medicine
Nutrigenomics has the potential to revolutionize preventive medicine by enabling proactive interventions to optimize health outcomes. By identifying genetic variations associated with increased disease risk, personalized nutrition recommendations can be developed to prevent or delay the onset of various conditions, such as cardiovascular diseases, type 2 diabetes, and certain cancers. Nutrigenomics can inform targeted interventions, such as dietary modifications, supplementation, or lifestyle changes, tailored to an individual’s genetic profile. The incorporation of nutrigenomics into preventive medicine has the potential to shift the paradigm from reactive disease management to proactive health optimization.
Role in Wellness Programs
Nutrigenomics can play a crucial role in wellness programs by providing individuals with personalized nutrition recommendations to support their overall health and well-being. Wellness programs aim to promote healthy lifestyles, improve quality of life, and prevent chronic diseases. By incorporating genetic information into wellness initiatives, individuals can receive tailored guidance on dietary and lifestyle choices that optimize their health outcomes. Nutrigenomics can identify genetic variations that influence nutrient requirements, metabolic pathways, or responses to specific dietary components, helping individuals make informed decisions about their nutrition and lifestyle choices.
This image is property of www.frontiersin.org.
Promoting Education and Awareness about Nutrigenomics
Importance of Public Education
Public education plays a crucial role in raising awareness about nutrigenomics and its potential benefits for personalized health and nutrition. Educating the public about the fundamental principles of nutrigenomics, including gene-diet interactions and epigenetic regulation, can empower individuals to make informed choices about their nutrition and overall health. Public education initiatives can help dispel misconceptions, highlight the evidence-based nature of nutrigenomics, and address concerns related to privacy, data protection, and genetic determinism. By promoting education and awareness, individuals can become active participants in their health journey and advocates for personalized nutrition.
Current Efforts and Successes
Numerous organizations and initiatives are working towards promoting education and awareness about nutrigenomics. Academic institutions, research institutions, and professional societies are incorporating nutrigenomics into their curricula and continuing education programs for healthcare professionals. Public health campaigns, online resources, and social media platforms provide accessible information about the principles and applications of nutrigenomics. Success stories and case studies highlighting the benefits of personalized nutrition can inspire individuals to explore nutrigenomics for optimizing their health outcomes. These efforts are contributing to a better understanding of nutrigenomics and its potential to revolutionize health and nutrition.
Potential Strategies for Increased Awareness
To further increase awareness about nutrigenomics, various strategies can be implemented. Collaboration between academic institutions, research organizations, healthcare providers, and policymakers is crucial for developing comprehensive and evidence-based educational materials. Public health campaigns targeting diverse populations and using culturally appropriate messaging can reach a wider audience. Incorporating nutrigenomics into school curricula or workplace wellness programs can provide individuals with foundational knowledge and empower them to make informed choices. Additionally, partnerships with community organizations, patient advocacy groups, and media outlets can help disseminate accurate and engaging information about nutrigenomics, fostering increased awareness and understanding.
Concluding Thoughts on Malnutrition, Epigenetics and Nutrigenomics
Reviewing the Interaction Between Malnutrition and Epigenetics
Malnutrition, whether due to inadequate nutrient intake or poor nutrient absorption, has a significant impact on epigenetic regulation. Nutritional deficiencies can lead to alterations in DNA methylation and histone modifications, affecting gene expression and potentially contributing to the development of various diseases. Conversely, optimal nutrition supports proper epigenetic regulation, facilitating normal development and reducing the risk of chronic diseases. The interplay between malnutrition and epigenetics highlights the importance of addressing nutrient deficiencies and promoting a balanced and nutrient-rich diet for optimal health outcomes.
Future Prospects and Research in Nutrigenomics
The field of nutrigenomics holds great promise for personalized nutrition and preventive medicine. Ongoing research efforts continue to uncover new genetic associations with dietary responses and health outcomes, expanding our understanding of gene-diet interactions. Advancements in technology, such as next-generation sequencing and artificial intelligence, are revolutionizing the field, enabling more comprehensive analyses and more accurate personalized nutrition recommendations. Longitudinal studies and large-scale cohort studies will provide critical insights into the long-term effects of gene-diet interactions on health and disease. Continued research in nutrigenomics will further drive the integration of personalized nutrition into healthcare systems and enhance our ability to optimize individual health outcomes.
Emphasizing the Need for Continued Research and Integration into Healthcare Systems
The intricate relationship between genetics and nutrition underscores the need for continued research and integration of nutrigenomics into healthcare systems. Nutrigenomics has the potential to revolutionize personalized nutrition, preventive medicine, and wellness programs, improving health outcomes and reducing the burden of chronic diseases. However, challenges related to ethics, accessibility, and the complexity of gene-diet interactions must be addressed. Education and awareness initiatives can empower individuals to make informed choices about their nutrition and advocate for personalized health solutions. With continued research and integration, nutrigenomics has the potential to transform healthcare and optimize individual health outcomes.




